Active Thermochemical Tables: Enthalpies of Formation of Bromo- and Iodo-Methanes, Ethenes and Ethynes | The Journal of Physical Chemistry A
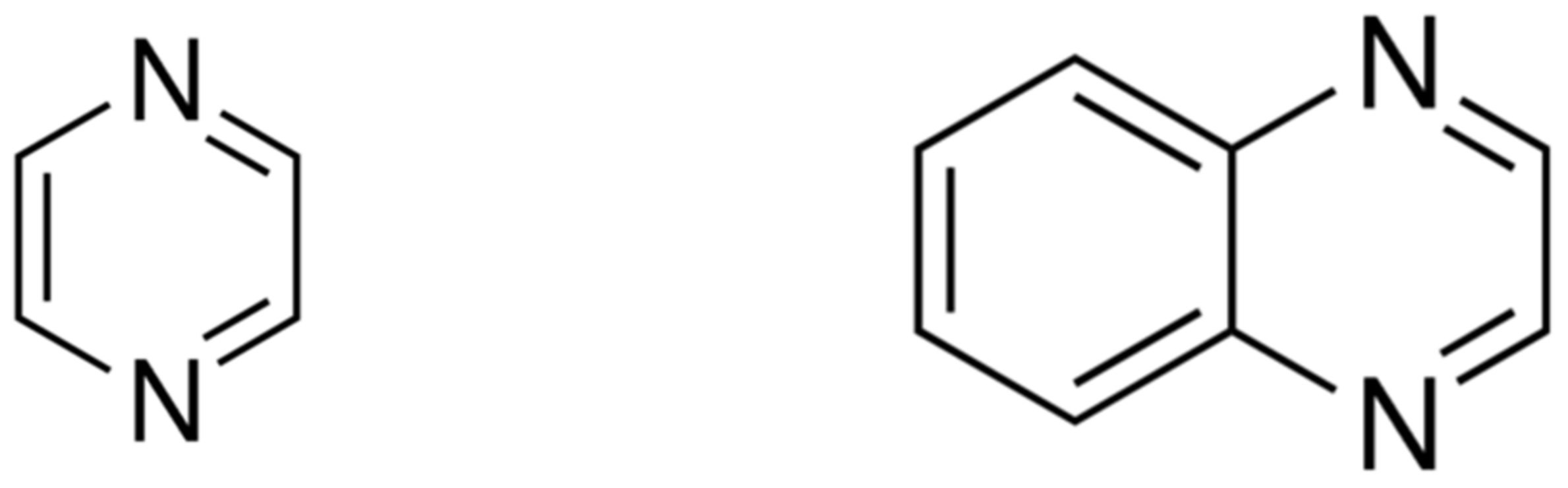
Hydrogen | Free Full-Text | Energetics of LOHC: Structure-Property Relationships from Network of Thermochemical Experiments and in Silico Methods

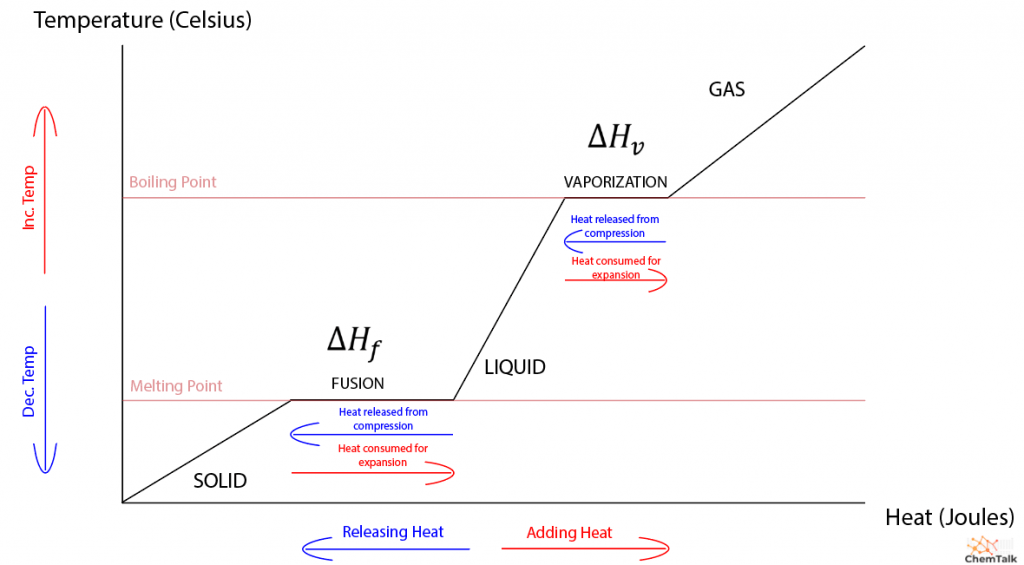
Enthalpy changes (ΔH in J g −1 ) associated with fusion (melting) of... | Download Scientific Diagram

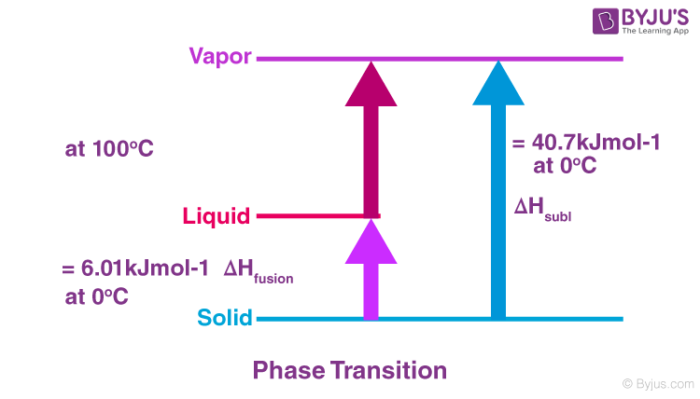
Latent heat of fusion of ice at 0^(@)C is 80cal/g. calculate the olar entropy change for the fusion process.
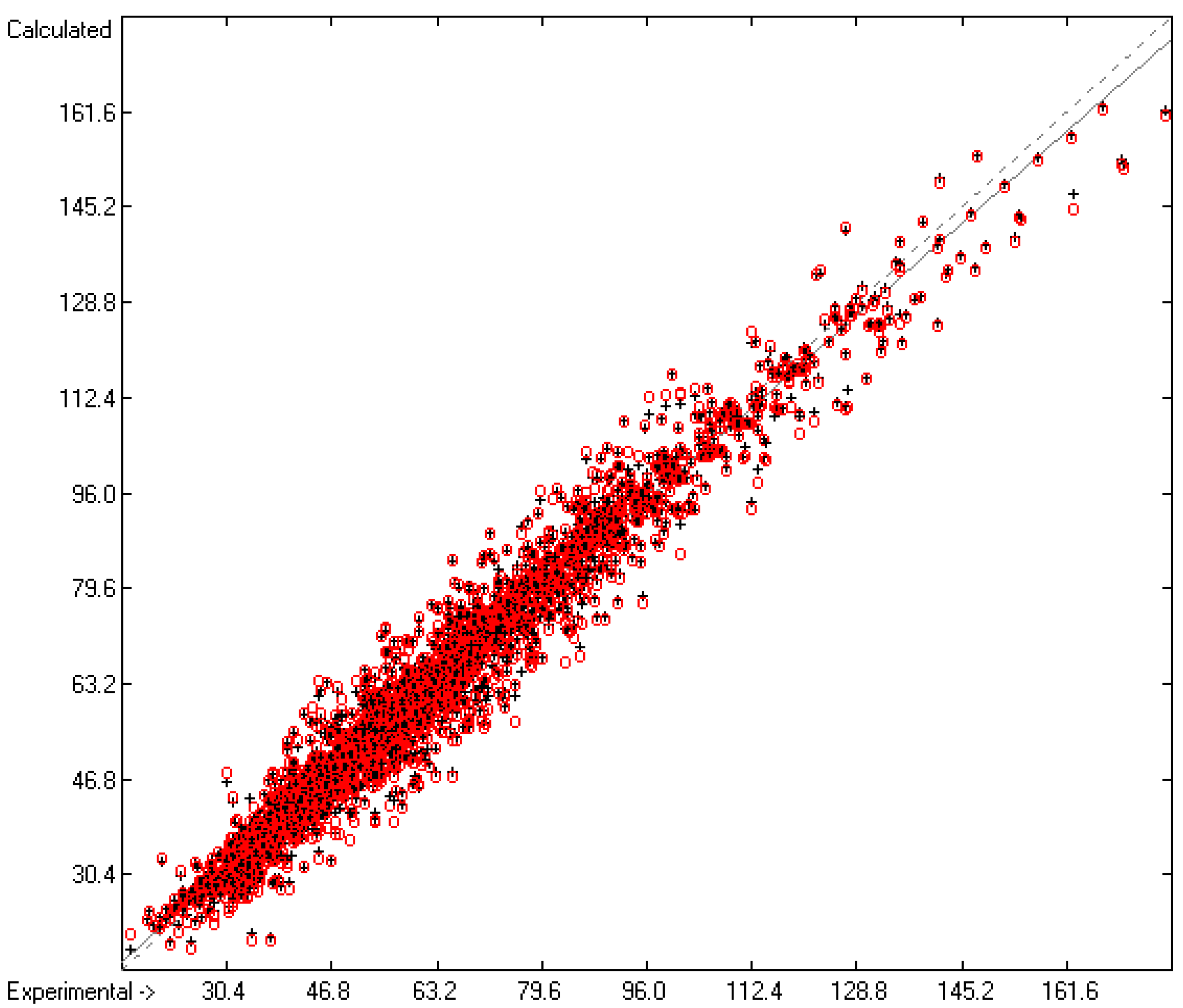
Molecules | Free Full-Text | Calculation of Five Thermodynamic Molecular Descriptors by Means of a General Computer Algorithm Based on the Group-Additivity Method: Standard Enthalpies of Vaporization, Sublimation and Solvation, and Entropy
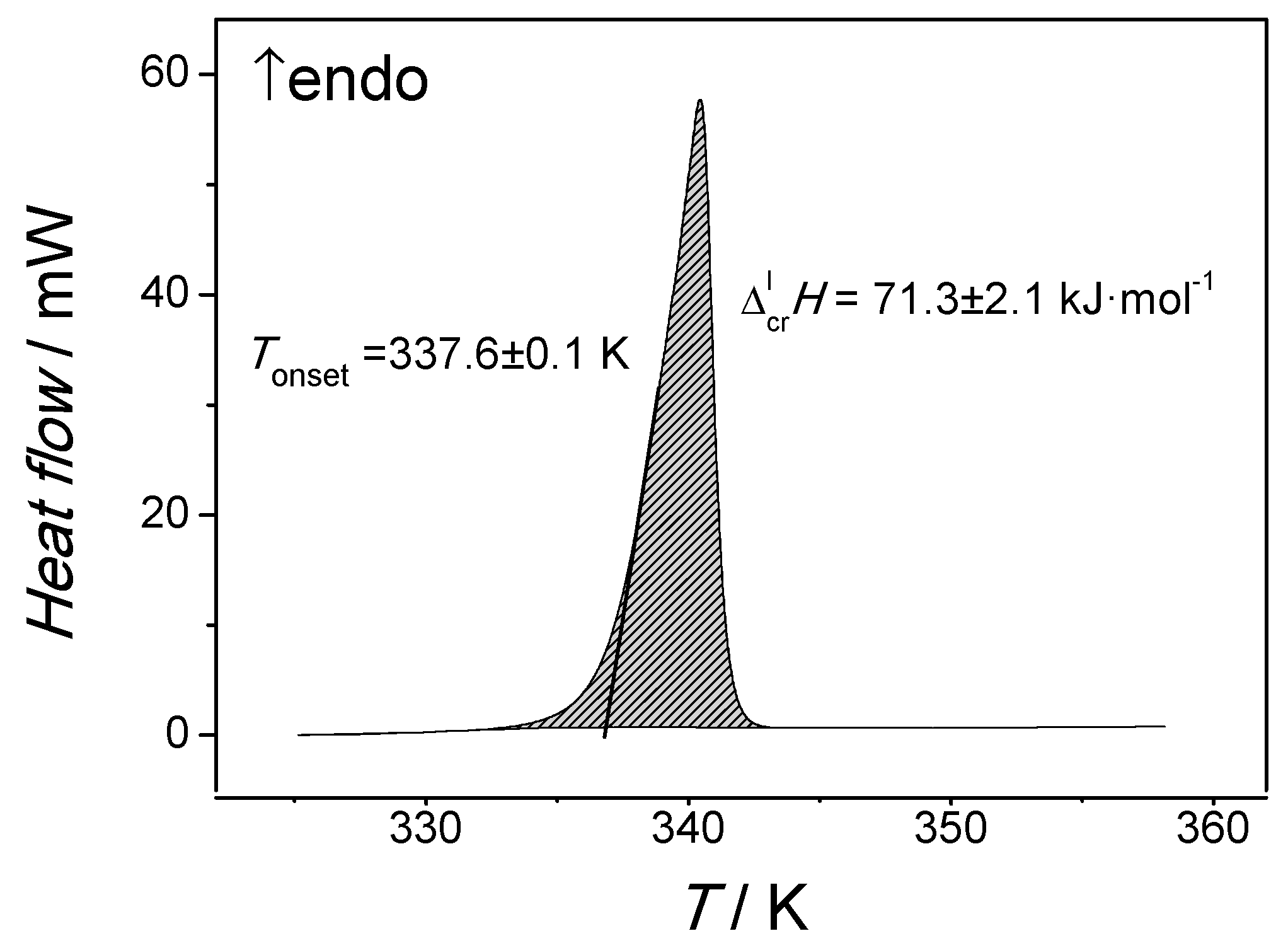
Liquids | Free Full-Text | Application of Solution Calorimetry to Determining the Fusion Enthalpy of an Arylaliphatic Compound at 298.15 K: n-Octadecanophenone

Modeling and Optimization of a Suspension Crystallization Separation Process for para-Xylene Purification | Organic Process Research & Development
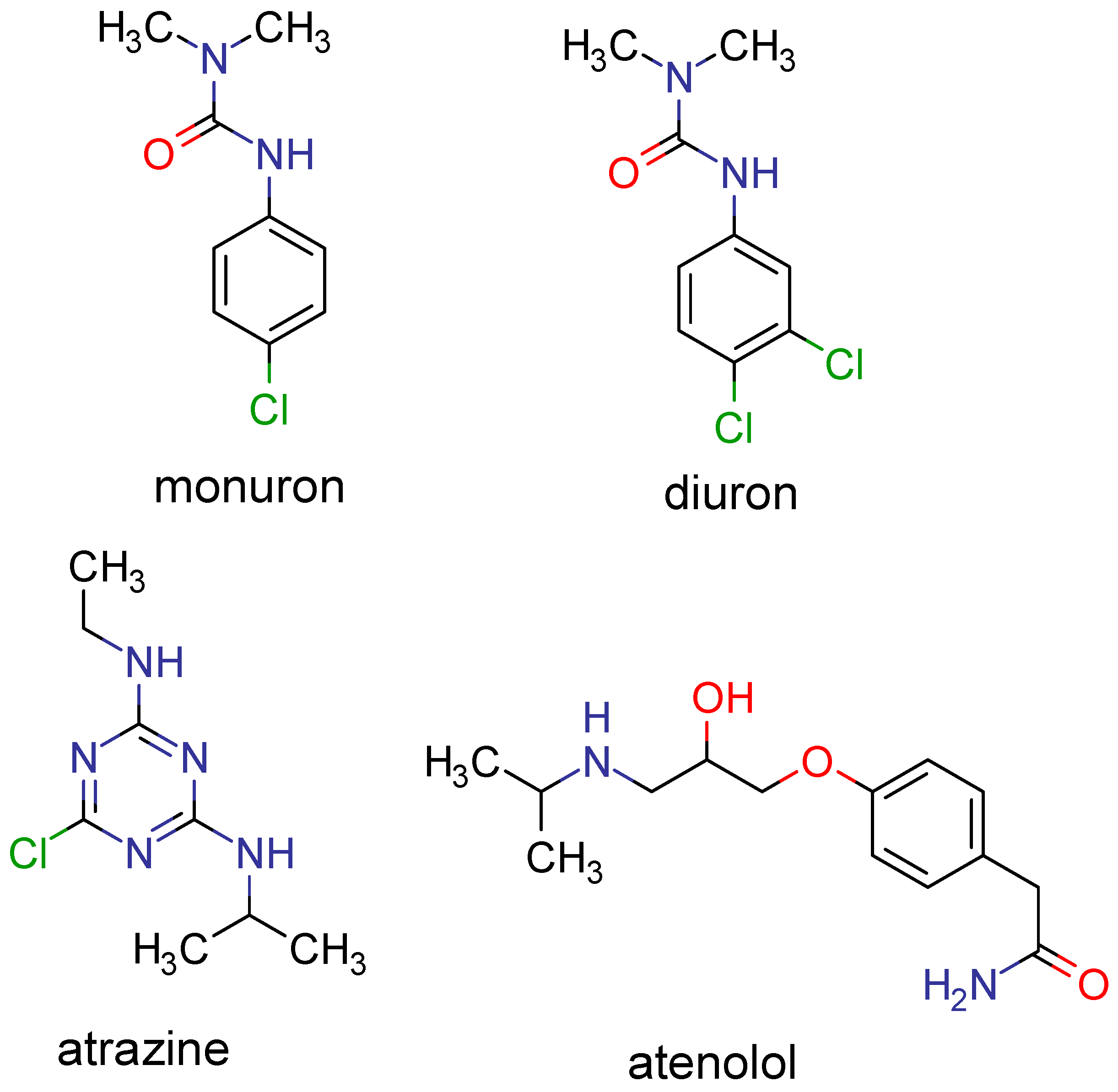
Processes | Free Full-Text | Predicting the Solubility of Nonelectrolyte Solids Using a Combination of Molecular Simulation with the Solubility Parameter Method MOSCED: Application to the Wastewater Contaminants Monuron, Diuron, Atrazine and

Energies | Free Full-Text | Insight into the Thermodynamic Properties of Promising Energetic HNTO·AN Co-Crystal: Heat Capacity, Combustion Energy, and Formation Enthalpy

Molecular Weight Dependence of Block Copolymer Micelle Fragmentation Kinetics | Journal of the American Chemical Society