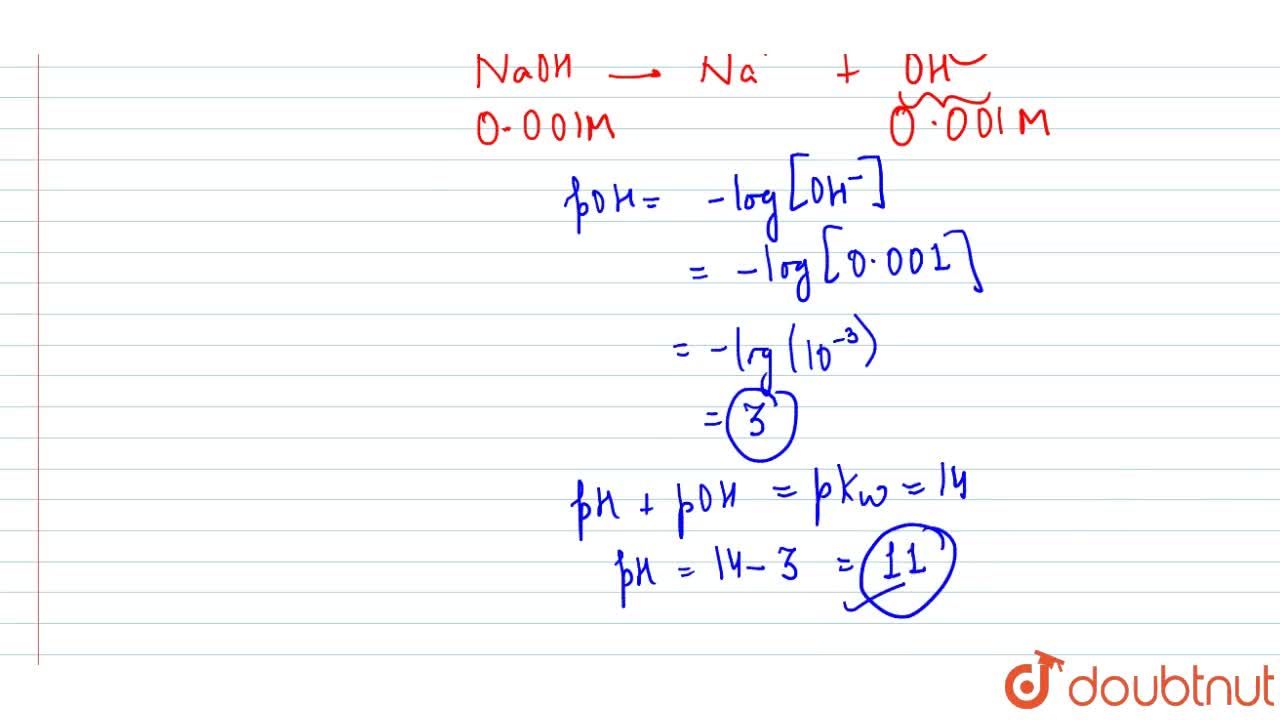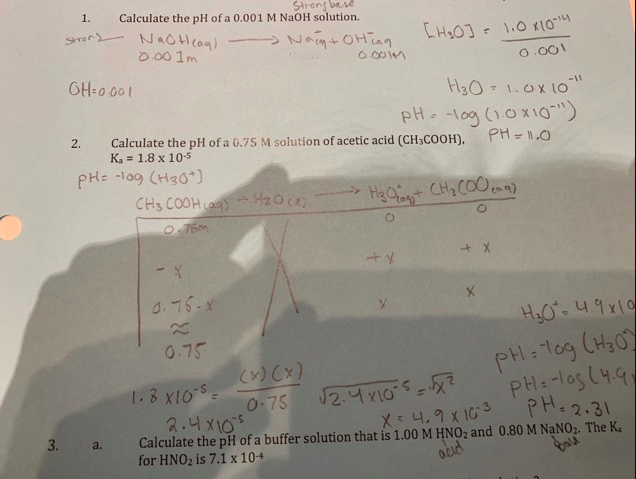What is the pH of the resulting solution when the equal volume of 0.1M Noah and 0.01M HCL are mixed? - Quora

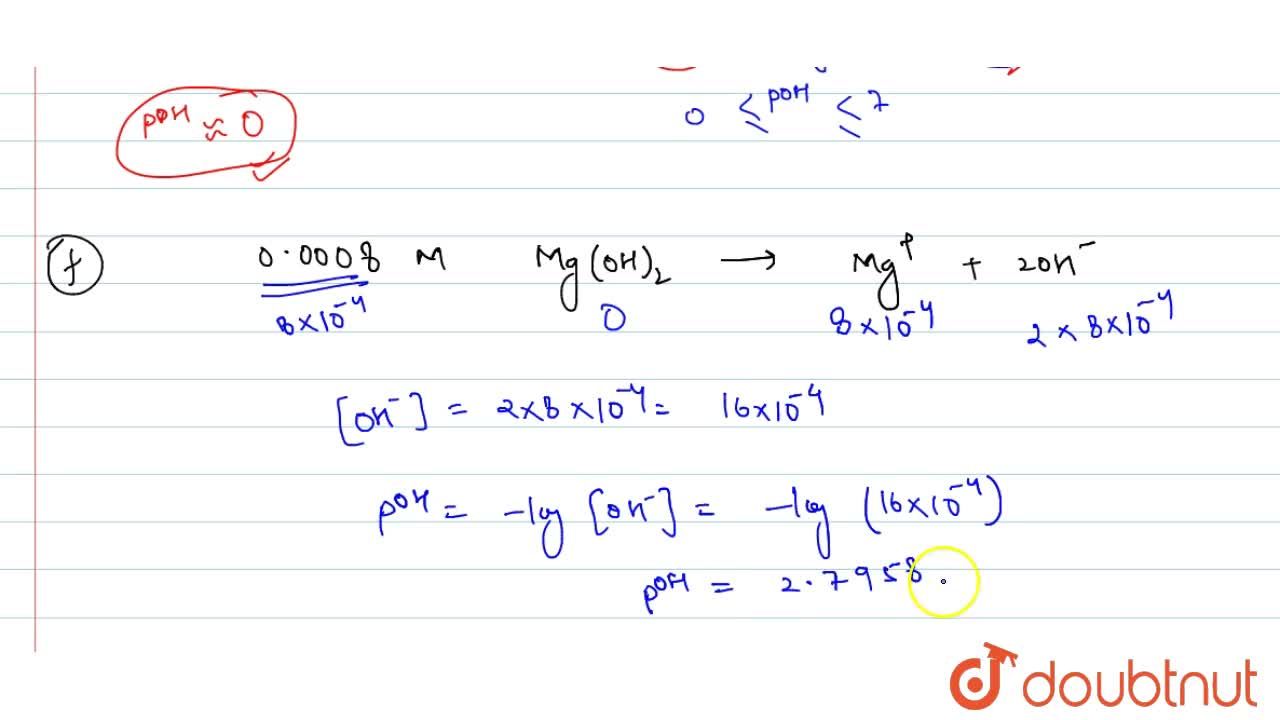
Calculate pH of the following solutions: (i) 0.001M HNO3 (ii) 0.005M H2SO4 (iii) 0.01M KOH (iv) 10^-8M NaOH (v) 0.0008M Ba (OH) 2
Calculate pH for : (a) 0.001 N NaOH, (b) 0.01 N Ca(OH)2 - Sarthaks eConnect | Largest Online Education Community
Calculate pH for : (a) 0.001 N NaOH, (b) 0.01 N Ca(OH)2 - Sarthaks eConnect | Largest Online Education Community

Calculate pH of the following solutions: (i) 0.001M HNO3 (ii) 0.005M H2SO4 (iii) 0.01M KOH (iv) 10^-8M NaOH (v) 0.0008M Ba (OH) 2

SOLVED: The pH of the solution prepared by dilution 1000 times of 0.001 M NaOH solution is: 12 10 A 500 mL of river water contains 0.06 g of mercury: the concentration

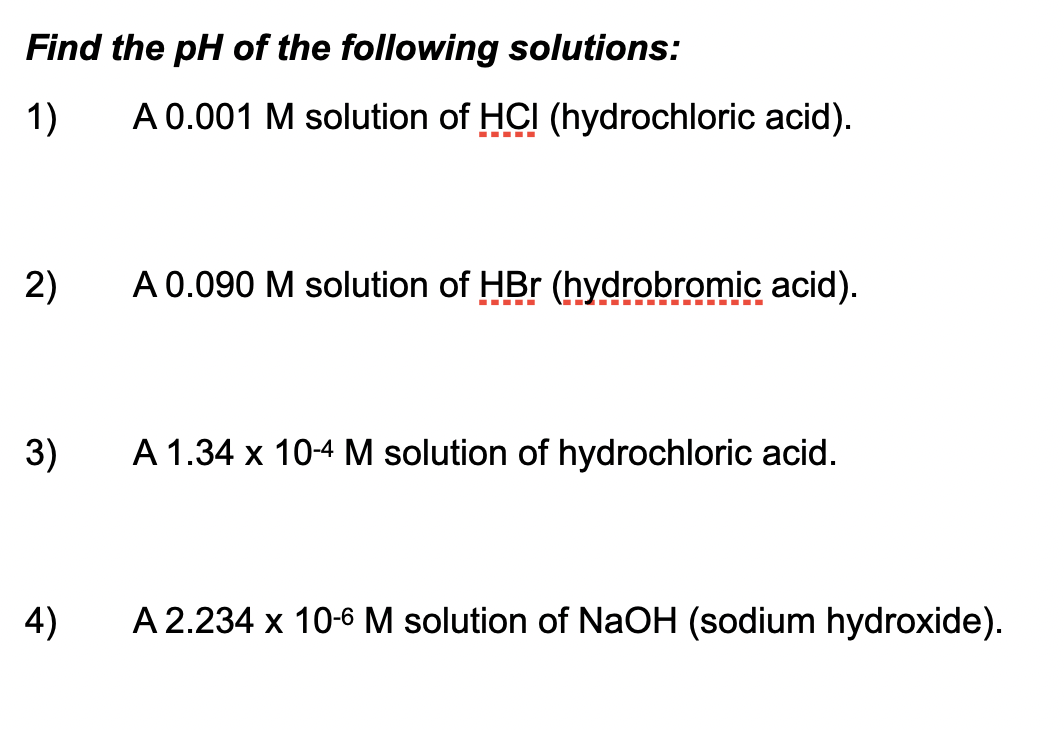
SOLVED: pH What is the pH of 0.0M HCI solution? Ans What is the pH of 0.02M HCL solution? Ans 1.7 What is the pH of 0.0[M NaOH solution? ZI 'SUV What
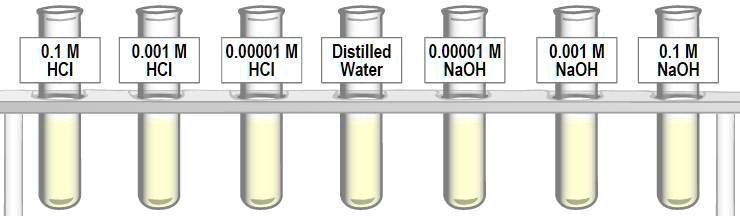
SOLVED: 'What are the pH of these solutions? 0.1 M HCI 0.001 M HCI 0.00001 M HCI Distilled Water 0.00001 M NaOH 0.001 M NaOH 0.1 M NaOH'